Antibody-Drug Conjugates
Antibody Drug Conjugates (ADCs) have emerged as a novel and effective modality in cancer therapeutics. ADCs are Monoclonal Antibodies (mAbs) to which cancer drugs are chemically attached.[1] The idea is to take advantage of the specificity provided by monoclonal antibodies to achieve targeted delivery of the drug to cancer cells. ADCs have been used at the lab level for decades, but some are now entering clinical use. Increasingly, these drugs herald a new modus operandi in the management of patients with cancer.
Pfizer’s Mylotarg™ (gemtuzumab ozogamicin) was the first ADC approved for therapeutic use in 2000; it was later pulled from the market but reapproved in 2017. [1, 2, 7] It was designed for the treatment of CD33 positive Acute Myeloid Leukemia (AML). The voluntary withdrawal followed confirmatory (Phase IV) clinical trials which demonstrated no improvement in overall survival rates in patients undergoing therapy with gemtuzumab. However, when more data cane in and showed benefits, the FDA again approved gemtuzumab. The European Medicines Agency has also approved us of this drug. [7]
How do ADCs Work?
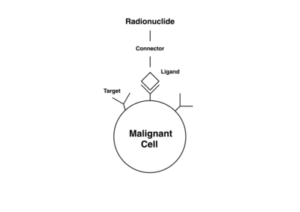 ADCs have been compared to target-bound missile systems to which a drug payload has been attached. [4] Indeed, they are sometimes called armed antibodies or empowered antibodies. The human immune system creates antibodies in response to microorganisms and other triggers, the body recognizes cancer cells ‘s immune system produces unique antibodies against cancer cells and the biochemical mediators they synthesize. These antibodies coat the surfaces of the cancer cells where they initiate complex cascades of reactions to kill the cancer cells [1] The concept of ADC rests on the fact that the specificity offered by monoclonal antibodies can be utilized to deliver payloads of cytotoxic cancer drugs mainly to the cancer cells. Once the ADC is inside the cancer cells, the cytotoxic drug payloads are cleaved off or released and subsequently exert their cell-killing effects. [1, 4]
ADCs have been compared to target-bound missile systems to which a drug payload has been attached. [4] Indeed, they are sometimes called armed antibodies or empowered antibodies. The human immune system creates antibodies in response to microorganisms and other triggers, the body recognizes cancer cells ‘s immune system produces unique antibodies against cancer cells and the biochemical mediators they synthesize. These antibodies coat the surfaces of the cancer cells where they initiate complex cascades of reactions to kill the cancer cells [1] The concept of ADC rests on the fact that the specificity offered by monoclonal antibodies can be utilized to deliver payloads of cytotoxic cancer drugs mainly to the cancer cells. Once the ADC is inside the cancer cells, the cytotoxic drug payloads are cleaved off or released and subsequently exert their cell-killing effects. [1, 4]
Therapeutic Advantages of ADCs
 ADCs are truly targeted. Conventional cancer drugs produce a wide arrays of side effects. This is because conventional chemotherapy agents kill rapidly proliferating cells, irrespective of whether the cells are malignant or healthy. Adverse effects such as neutropenia, anemia, and thrombocytopenia result from damage to the bone marrow. The effect on follicular cells in the scalp results in hair loss. The major difference between ADCs and conventional cancer drugs is specificity. [5, 6, 7] Unlike conventional cancer drugs, ADCs target only the cancer cells. The monoclonal antibodies bind specifically to the malignant cells. Since only the malignant cells are targeted, ADCs produce fewer side effects compared to conventional drugs. The dose per cancer cell can be high because there is less worry about side effects.
ADCs are truly targeted. Conventional cancer drugs produce a wide arrays of side effects. This is because conventional chemotherapy agents kill rapidly proliferating cells, irrespective of whether the cells are malignant or healthy. Adverse effects such as neutropenia, anemia, and thrombocytopenia result from damage to the bone marrow. The effect on follicular cells in the scalp results in hair loss. The major difference between ADCs and conventional cancer drugs is specificity. [5, 6, 7] Unlike conventional cancer drugs, ADCs target only the cancer cells. The monoclonal antibodies bind specifically to the malignant cells. Since only the malignant cells are targeted, ADCs produce fewer side effects compared to conventional drugs. The dose per cancer cell can be high because there is less worry about side effects.
ADCs available for cancer treatment
PDF List of Conjugates.
Polatuzumab vedotin
Brand/Trade Names: Polivy
Formula: C6670H10317N1745O2087S40
Antibody Origin: Humanized G1
Mechanism: Binds to CD30
Toxin: Auristatin, a microtubule destabilizer also called vedotin.
Administration: Intravenous
Notes: Approved by the FDA in 2019. Used for treatment of large B-cell lymphoma.
Enfortumab vedotin-ejfv
Brand/Trade Names: Padcev
Formula:
Antibody Origin: Human G1
Mechanism: anti-Nectin-4
Toxin: Auristatin, a microtubule destabilizer
Administration: Intravenous
Notes: Approved by the FDA in 2019. Used for treatment of bladder cancer (urothelial cancer).
Fam-trastuzumab deruxtecan-nxki
Brand/Trade Names: Enhertu
Formula:
Antibody Origin: Humanized
Mechanism: anti-HER2
Toxin: topoisomerase I inhibitor
Administration: Intravenous
Notes: Approved by the FDA in 2019. Used for treatment of breast cancer.
Gemtuzumab ozogamicin
Brand/Trade Names: Mylotarg, Gemtuzumab Ozogamicin
Formula:
Antibody Origin: Humanized G4
Mechanism: Binds to C33
Toxin: calicheamicin (antibiotic)
Administration: Intravenous
Notes: Approved by FDA in 2017. Used for treatment of acute myeloid leukemia.
Brentuximab vedotin
Brand/Trade Names: Adcetris
Formula: C6476H9930N1690O2030S40 (C68H105N11O15)3–5
Antibody Origin: Chimera (human/mouse) G1
Mechanism: Binds to CD30
Administration: Intravenous
Notes: Approved by the FDA in 2011. Approved for treatment of peripheral T-cell lymphoma, cutaneous T-cell lymphoma, cutaneous anaplastic large cell lymphoma, Hodgkin’s Disease, anaplastic large cell lymphoma.
Tisotumab vedotin-tftv
Brand/Trade Names: Tivdak
Formula:
Origin: Human G1
Mechanism: Binds to CD142
Toxin: Auristatin, a microtubule destabilizer
Administration: Intravenous
Notes: Approved by the FDA in 2021 for treatment of cervical cancer. First approved drug that targets tissue factor.
Ado-Trastuzumab emtansine
Brand/Trade Names: Kadcyla, Ado-Trastuzumab emtansine
Formula: C6448H9948N1720O2012S44·(C47H62ClN4O13S)n
Antibody Origin: Humanized G1
Mechanism: anti-HER2
Toxin: Mertansine, a microtubule inhibitor also called DM1.
Administration: Intravenous
Notes: Approved by the FDA in 2019. First ADC ever approved by the FDA for the treatment of a solid tumor. Used for treatment of HER2 positive metastatic breast cancer. [1, 7]
Inotuzumab ozogamicin
Brand/Trade Names: Besponsa
Formula: C6518H10002N1738O2036S42
Antibody Origin: Humanized G4
Mechanism: Binds to CD22
Toxin: calicheamicin derivative, N-acetyl-gamma-calicheamicin dimethylhydrazide
Administration: Intravenous
Notes: Approved by FDA in August 2017. Used for the treatment B-cell non-Hodgkin lymphoma.
Denileukin diftitox
Brand/Trade Names: Ontak
Formula: C2560H4042N678O799S17
Antibody Origin: N/A
Mechanism: Binds to CD22.
Toxin: Diphtheria toxin (DT388)
Administration: Intravenous
Notes: Approved by the FDA in 1999. Used for treatment of leukemia and lymphoma. No longer marketed in the US.
Tagraxofusp-erzs
Brand/Trade Names: Elzonris
Formula:
Antibody Origin: N/A
Mechanism: Binds to CD123/interleukin 3 (IL-3) receptor α (IL-3Rα)
Toxin: Diphheria toxin (DT388)
Administration: Intravenous
Notes: Interleukin 3 (IL-3) fused to diphtheria toxin. Approved by the FDA in 2018. Used for treatment of blastic plasmacytoid dendritic cell neoplasm.
Sacituzumab govitecan-hziy
Brand/Trade Names: Trodelvy
Formula: C76H104N12O24S
Antibody Origin: Humanized G1
Mechanism: anti-TROP-2
Toxin: topoisomerase 1 inhibitor SN38
Administration: Intravenous
Notes: Approved by the FDA in 2020. Used for treatment of breast cancer.
Moxetumomab pasudotox-tdfk
Brand/Trade Names: Lumoxiti
Formula: C2804H4339N783O870S14
Origin: Mouse G1
Mechanism: Anti-CD22
Toxin: PE38 (fragment of Pseudomonas exotoxin A)
Administration: Intravenous
Notes: Approved in Sept 2018 under FDA fast-track designation. Orphan drug status. Used for treatment of hairy cell leukemia.
Loncastuximab tesirine
Brand/Trade Names: Zynlonta
Formula: C6544H10048N1718O2064S52
Origin: Humanized G1
Mechanism: Anti-CD19
Toxin: pyrrolobenzodiazepine dimer
Administration: Intravenous
Notes: Approved in 2021 for treatment of lymphoma.
Mirvetuximab soravtansine-gynx
Brand/Trade Names: Elahere
Formula:
Origin: Chimeric G1
Mechanism: Anti-FRa
Toxin: DM4 (maytansinoid microtubule inhibitor)
Administration: Intravenous
Notes: Approved in 2022 for treatment of ovarian cancer.
Conjugates for Radioimmunotherapy
Radioimmunotherapy for cancer involves use of an antibody connected with a radionuclide. The antibody has a specificity for the tumor cells. This strategy delivers a radioactive atom to the malignant cell, where it is hoped the radiation will kill the cell. The atoms used emit Beta radiation, which can kill a cell at close range, but it not as destructive to the tissue as Gamma radiation. These combinations are sometimes called radioconjugates.
Like other medical treatment, radioimmunotherapy exploits something that is different about the diseased tissues, in this case malignant cells. When the tumor cells express an antigen that healthy cells do not, there is an opportunity for an immunotherapy approach.
Four radioimmunotherapy conjugates were approved, and three are still on the market.
Bexxar was a conjugate of tositumomab and Iodine-131. It was approved by the FDA in 2003 and withdrawn in 2014. Zevalin is a conjugate of Ibritumomab and Yttrium-90. Lutathera is a conjugate of Lutetium-177 and dotatate. The newest radioummunotherapy conjugate, Pluvicto, is a conjugate of Lutetium-177 and vipivotide tetraxetan. It was approved by the FDA in 2022.
Ibritumomab tiuxetan
Brand/Trade Names: Zevalin
Formula:
Antibody Origin: Mouse G1
Mechanism: Binds to CD20
Toxin: radioactive Yttrium-90 or Indium-111
Administration: Intravenous
Notes: Approved by the FDA in 2002. Used for treatment of B-cell non-Hodgkin’s lymphoma. Indium-111 (111In) has a half-life of 2.8 days, while Yttrium-90 (90Y) has a half-life of 2.7 days.
Lutetium Lu 177 Dotatate
Brand/Trade Names: Lutathera
Formula: C85H90N14O19S2
Antibody Origin: N/A
Mechanism: Binds to Somatostatin receptors
Toxin: radioactive Lutetium-177, aka 177Lu
Administration: Intravenous
Notes: Approved by the FDA in 2018. Also called Lutetium oxodotreotide. Used for treatment of gastroenteropancreatic neuroendocrine tumors. Investigated for treatment of hepatocellular carcinoma.
Lutetium Lu 177 vipivotide tetraxetan
Brand/Trade Names: Pluvicto
Formula:
Antibody Origin: N/A
Mechanism:
Toxin: radioactive Lutetium-177, aka 177Lu
Administration: Intravenous
Notes: Approved by the FDA in 2022. Used for treatment of prostate cancer. Also called LuPSMA and 177Lutetium-PSMA-617
Tositumomab
Brand/Trade Names: Bexxar
Formula: C6416H9874N1688O1987S44
Antibody Origin: Mouse
Mechanism: Binds to CD22
Toxin: I-131 radioactive isotope, aka 131I
Administration: Intravenous
Notes: Approved by the FDA in 2003. Later withdrawn from market and no longer used. Used to treat lymphoma. I-131 has a half-life of 8 days and emits Beta radiation.
Common Conjugates
Vedotin is Monomethyl auristatin E (MMAE) – C39H67N5O7. This agent works in M-phase of the cell cycle and stops division. By itself it is too toxic for use, but as part of an ADC they are valuable.
The calicheamicins are antibiotics from nature. One is ozogamicin.
Emtansine is Mertansine (aka DM1). Mertansine is a tubulin inhibitor, meaning that it also works in the M-phase and inhibits the division of the cell nucleus.
References
- H.L. Perez, et al., Antibody-drug conjugates: current status and future directions, Drug Discov Today (2013), http://dx.doi.org/10.1016/j.drudis.2013.11.004
- FDA Approval For Gemtuzumab Ozogamicin: Reintroduction Based on Favorable Risk, ADC Reviews (September 2017), https://adcreview.com/news/fda-approval-gemtuzumab-ozogamicin-reintroduction-based-favorable-riskbenefit-profile/
- Antibody-Drug Conjugates: Technologies and Global Markets, ( June 2017), https://www.reportlinker.com/p02042686/Antibody-Drug-Conjugates-Technologies-and-Global-Markets.html#utm_source=prnewswire
- Ojima Iwao, Guided Molecular Missiles for Tumor-Targeting Chemotherapy—Case Studies Using the Second-Generation Taxoids as Warheads, (2008), https://pubs.acs.org/doi/abs/10.1021/ar700093f
- Reichert J.M., Dhimolea E. The future of antibodies as cancer drugs. Drug. Discov. Today. 2012;17:954–963. Doi :10.1016/j.drudis.2012.04.006 https://www.researchgate.net/publication/224914425_The_future_of_antibodies_as_cancer_drugs
- Schrama D., Reisfeld R.A., Becker J.C. Antibody targeted drugs as cancer therapeutics. Nat. Rev. Drug Discov.,(2006) https://www.ncbi.nlm.nih.gov/pubmed/16424916
- What are Antibody-Drug Conjugates? ADC Reviews (2017), https://adcreview.com/adc-university/adcs-101/antibody-drug-conjugates-adcs/
- Mukherjee Sy, The FDA Just Approved a New Pfizer Cancer Drug for a rare, Vicious Leukemia, Fortune (2017), http://fortune.com/2017/08/18/fda-leukemia-pfizer-besponsa
 Today’s arsenal of chemotherapy agents includes many different classes of medicines. Researchers continue to find and test new drugs.
Today’s arsenal of chemotherapy agents includes many different classes of medicines. Researchers continue to find and test new drugs.