Workplace Hazards
More than other medicines, cancer drugs can be dangerous. Not to the patient, necessarily, but to others who handle or come into contact with them. Indeed, some chemotherapy agents actually cause cancer and/or birth defects. The federal government estimates “about 8 million U.S. healthcare workers are potentially exposed to hazardous drugs.” Medical personnel who handle these drugs should take safety precautions to avoid or minimize exposure.
A big hospital might have more hazardous chemicals than any other common place of employment. These hazards include CTX medicines. Jobs that can bring exposure to antineoplastic agents include physician, physician’s assistant, various kinds of nurses, pharmacists, drug couriers, custodial workers in hospitals (including anyone who handles waste), and shipping and receiving workers at hospitals. As veterinarians increasingly use oncology drugs, they and their technicians are at risk.
Pharmacists who prepare drug formulations and nurses who prepare and/or administer them are the two occupational groups who have the highest potential exposure to antineoplastic agents. Additionally,  physicians and operating room personnel may also be exposed through the treatment of patients. Hospital staff, such as shipping and receiving personnel, custodial workers, laundry workers and waste handlers, all have potential exposure to these drugs during the course of their work. Hygienists have found contamination of surfaces in pharmacies and nursing administration areas.
physicians and operating room personnel may also be exposed through the treatment of patients. Hospital staff, such as shipping and receiving personnel, custodial workers, laundry workers and waste handlers, all have potential exposure to these drugs during the course of their work. Hygienists have found contamination of surfaces in pharmacies and nursing administration areas.
The side effects that chemotherapy drugs produce in cancer patients show up in healthcare workers who do not have cancer but who have been exposed to the medicines, These include nausea, anemia, infertility, and hair loss. The drugs can also increase the problems with fetal development and pregnant workers need to be especially vigilant.
Industrial hygienists worry about employee safety and develop programs to reduce risk. Well-managed treatment center have equipment and procedures to protect people. Personal protective equipment is abbreviated PPE. The facilities are often subject to inspection by OSHA and state or regional regulatory agencies. Such equipment as impermeable gloves, facemasks, aprons, and needles designed for safety can be part of a chemotherapy medicine management plan. To limit risk of exposure new delivery systems called closed system drug transfer devices have been developed. These are designed to prevent leaks and to be airtight. Unfiltered air cannot enter and any exhaust is contained.
Even though sometimes the effects of the medicines on healthcare workers is not apparent in the short run, chronic low-level exposure can lead to inner ear damage (hearing loss), liver damage, kidney damage, and effects on the blood forming ability of the body and on the heart.
Occupational exposure to anti-cancer drugs causes health concerns in workers. Acute exposure can produce effects like side effects seen in patients: hair loss, skin problems, ototoxicity, nausea, and liver, kidney heart muscle damage. Chronic exposure produces effects on fertility although it has not been studied extensively.
Hospitals and oncology clinics should keep a list of medicines that can be hazardous to employees. Indeed, OSHA mandates creation of this list and that it be made available to workers and regulators. When a new drug is added, the list should be updated. Be sure to keep track of off-formulary drugs purchased by all parts of the organization, including, say, radiology. Managers of organizations that carry stocks of hazardous drugs should also be worried about generation and disposal of hazardous waste.
Monitoring of healthcare workers
There is no standard monitoring of healthcare workers who work with chemotherapy drugs as far as we know. This stands in contrast with the nuclear materials and nuclear power industries where workers are checked regularly, Some employers may have programs to measure employee exposure and investigators have used a number of biological endpoints to monitor exposure.
One method of checking involves running urine from workers through a bacterial mutagenicity assay (Ames test) that is sensitive to many of the drugs or their breakdown products. Chromosomal damage in white blood cells is also evidence of exposure to mutagenic material, although finding this requires a special blood test, Sister Chromatid Exchanges (SCEs) are also observed in lymphocytes by some scientists investigating occupational exposure of cytotoxic materials but it is not used widely in monitoring.
The Comet assay and old fashioned alkaline elution, can be used by trained technicians to find chromosomal damage, but we are not aware of their use in occupational exposure monitoring.
Protection
To protect everyone, mixing and other preparation of chemotherapy is often done in biological safety cabinets. These units are typically sized for one worker at a time. They have exhaust and ventilation systems and direct the air through a HEPA (high-efficiency particulate air) filter.
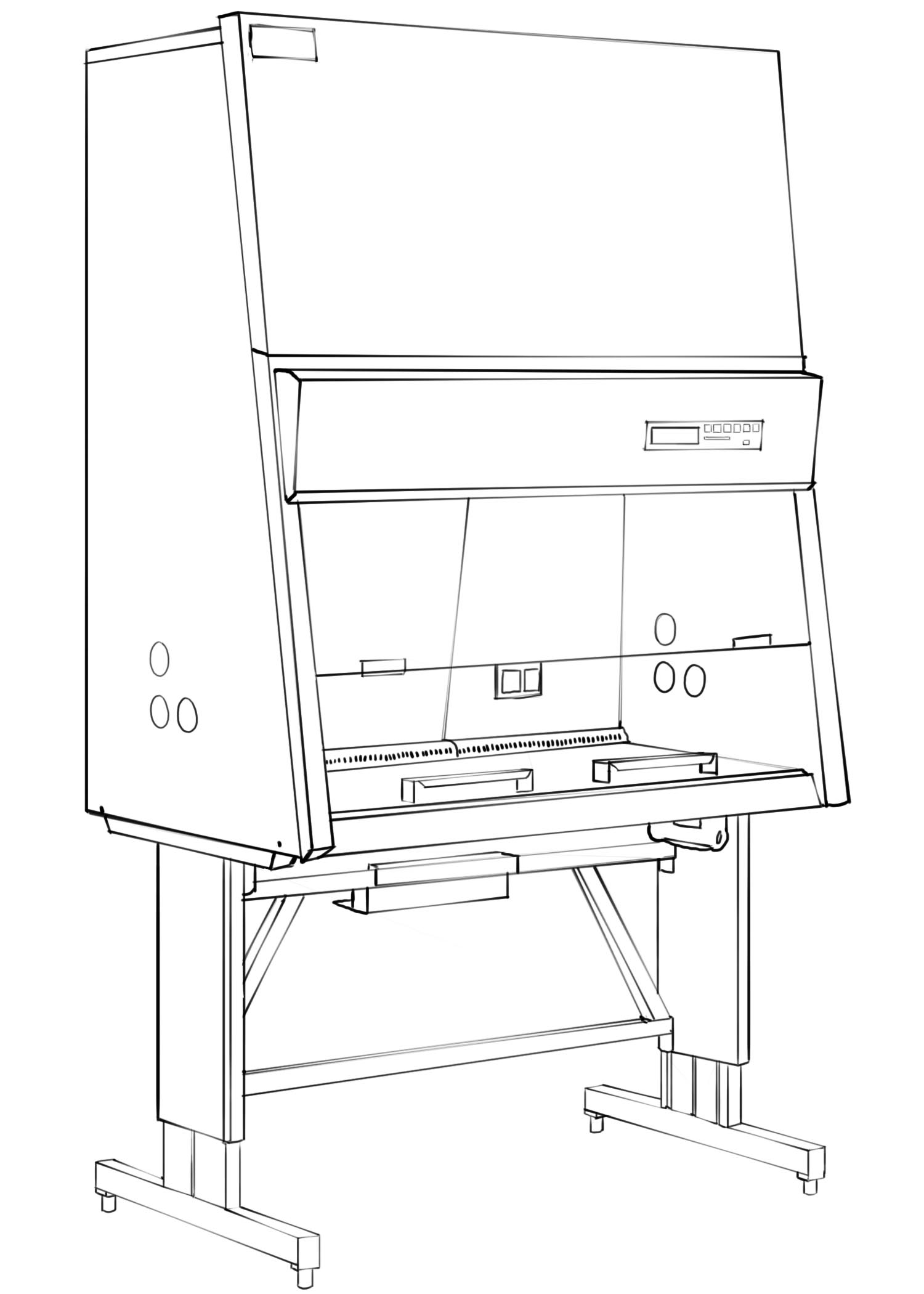
Biological Safety Cabinet
These nominally look like fume hoods and like work areas used in clean room environments, but they are different. Special gloves are recommended for handling chemotherapeutic agents. The American Society for Testing and Materials (ASTM) has standards for such gloves. Special gowns are designed for chemotherapy handling. They are usually plastic (polypropylene) and can be incinerated when classified as waste. Other types of personal protective equipment are facemasks and respirators.
Practices
Sharps
If you get cut by a sharp that has been entered into a medical waste stream (i.e, any medical waste), wash the exposed area with water and soap or use an antiseptic such as rubbing alcohol or hand sanitizer. Seek medical attention. This is particularly important if you know for sure the sharp has been used (e.g. the needle has been employed in treatment). Keep the sharp in case someone wants to analyze it.
There is also concern that accidental exposure is being underreported by nurses. There has been an increase in needle safety awareness in recent years, but it is not clear that awareness of chemical hazards is as high as it should be.
External resources: 2016 Updated American Society of Clinical Oncology / Oncology Nursing Society Chemotherapy Administration Safety Standards
OSHA’s website on controlling exposure to hazardous drugs.
ONS: The Case of the Explicit Exposure
Waste Considerations
Chemotherapy waste is a problem for health care industry. It’s less of a problem for individual patients who use chemo at home as the regulations and laws are written to exclude home use from many restrictions.
Chemo drugs and packaging can become waste if the drug is somehow adulterated or expires or becomes otherwise unusable. Other materials (e.g. delivery equipment) that comes into contact with chemo agents may pose special waste management challenges. Even trace levels of the medicines can render materials hazardous waste.
Hygienists have found that trace chemotherapy contamination poses a threat to workers. Many healthcare facilities have autoclaves onsite for the purpose of sterilizing equipment. But the chemical hazard of chemotherapy is not the same as the hazard posed by infectious agents. Despite the tendency of hospital personnel to throw everything into the autoclave, materials with chemo agents – even in residue amounts – must be disposed of as chemical waste. Usually proper management calls for incineration.
The World Health Organization publishes methods for chemical treatment of some chemotherapy agents with sulfuric acid and potassium permanganate, with sodium hypochlorite, with sodium hydroxide and potassium permanganate, and with sodium thiosulfate.
RCRA Hazardous waste
Under US federal law (the Resource Conservation and Recovery Act), there are two ways something can be classified as a hazardous waste
- Containing compounds explicitly named in the federal register when the Resource Conservation and Recovery Act was passed 40 CFR 261.33(f) in 1976. These are called listed wastes.
- Having enumerated characteristics – namely ignitable, corrosive (very high or low pH), reactive, or toxic. These are called characteristic wastes.
The explicitly named hazardous materials are on four lists, called by the letters F, K, P, and U. Listed wastes include spent materials (F– and K–list wastes) and commercial chemical products (U– and P-list wastes). At the time RCRA was enacted, there weren’t as many chemotherapy drugs in use as there are today. Only 9 agents are listed but over a hundred cancer drugs used today meet the definition of RCRA hazardous waste because they fit the characteristics (typically the toxic characteristic.) These include the vinca alkaloids and 5-fluorouracil.
Listed chemotherapy medicines:
| Medicine | RCRA Designation | |
| Arsenic Trioxide | P-012 | |
| Chlorambucil | U-035 | |
| Cyclophosphamide | U-058 | |
| Daunomycin | U-059 | |
| Diethystilbestrol | U-089 | |
| Melphalan | U-150 | |
| Mitomycin C | U-010 | |
| Streptozotocin | U-206 | |
| Uracil Mustard | U-237 |
 Today’s arsenal of chemotherapy agents includes many different classes of medicines. Researchers continue to find and test new drugs.
Today’s arsenal of chemotherapy agents includes many different classes of medicines. Researchers continue to find and test new drugs.