Checkpoint Inhibitors for Cancer Treatment
Some cancer cells have the capacity to deactivate the T lymphocytes (T cells) of the immune system. (Indeed, evasion of the immune system is one of the hallmarks of cancer.) Checkpoint inhibitors were developed to get around this problem. Inside the body, checkpoint inhibitors promote recognition of cancer cells as foreign cells by the immune system. They stop the inhibition of the immune system that allows cancer to grow.
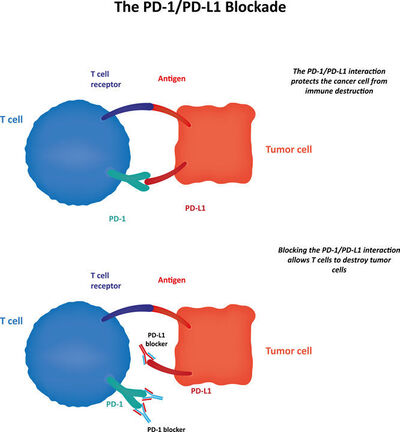 Under normal circumstances, T cells are not triggered to attack healthy cells because healthy human cells carry a protein known as Programmed death-ligand 1 (PD-L1). When a the checkpoint protein programmed cell death protein 1 (PD-1) on the T cell can bind to the PD-L1 of a normal cell, the immune system recognizes the normal cell as being non-pathogenic. Yet some cancer cells also carry large amounts of PD-L1 and so evade attack from the immune system simply because the cells are not recognized as malignant.
Under normal circumstances, T cells are not triggered to attack healthy cells because healthy human cells carry a protein known as Programmed death-ligand 1 (PD-L1). When a the checkpoint protein programmed cell death protein 1 (PD-1) on the T cell can bind to the PD-L1 of a normal cell, the immune system recognizes the normal cell as being non-pathogenic. Yet some cancer cells also carry large amounts of PD-L1 and so evade attack from the immune system simply because the cells are not recognized as malignant.
Immune checkpoint blockers are monoclonal antibodies which disable either the PD-1 of the T cell or the PD-L1 protein on other cells preventing the binding from taking place. This leaves the immune system free to attack the malignant cell.
Another protein which can be present in certain cancer cells is cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). Ipilimumab has been developed to prevent this protein from binding to immune cells. However, an article in Nature in 2022 stated that the pharmaceutical industry has spent over $100 billion in research and development of checkpoint inhibitors.
Immune checkpoint inhibitors are an important advance in cancer treatments, and several drugs in use today target either the PD-1 checkpoints or the PD-L1 proteins. The most commonly used are:
PD-1
Pembrolizumab (Keytruda)
Nivolumab (Opdivo)
Cemiplimab-rwlc (Libtayo)
Tislelizumab-jsgr (Tevimbra)
Dostarlimab-gxly (Jemperli)
PD-L1
Atezolizumab (Tecentriq)
Avelumab (Bavencio)
Durvalumab (Imfinzi)
CTLA-4
Ipilimumab (Yervoy)
The inhibitory immune checkpoint lymphocyte activation gene-3 (LAG-3) is also of interest to drug developers, and a combination of Relatlimab and Nivolumab is thought to target both PD-1 and LAG-3.
Do checkpoint inhibitors work? In some cases they are very effective. The discovery of checkpoint inhibitors was considered a breakthrough in understanding the mechanics of cancer progression. Like other cancer treatments, they are more effective in some cases than others.
Currently, these treatments are generally employed only against cancers which are known to carry the PD-L1 and CTLA-4 binding proteins, namely advanced melanoma, which is unresectable or metastatic, non-small cell lung cancer, head and neck squamous cell carcinoma, and bladder cancer. Trials are also underway to find out if they work on other types of cancers. Checkpoint inhibitors have shown scientists that the immune system can be stimulated to recognize malignant cells, and opened up research to other mechanisms that can induce anticancer immunity.
Combinations are also a possibility and one trial showed melanoma patients treated with nivolumab and ipilimumab had good results. Kinase inhibitors are considered good candidates for combination with checkpoint inhibitors. Kinase oncogenes may play a role in allowing some cancers to escape the immune system, and allowing tumors to resist T-cell checkpoint inhibitors. Kinase inhibitors could therefore be part of a lethal one-two punch to cancer when combined to the PD-1, PD-L1, and CTLA-4 inhibitors.
Obstacles
These immunotherapy treatments essentially prevent protein binding not only to pathogenic cells but also to healthy ones, which can cause side effects. Sometimes the checkpoint inhibitors become a double-edged sword as the T-cells attack and disrupt the normal function of healthy organs and tissues.
Unfortunately, it is difficult to predict how frequently and severe such issues will be. Although few deaths have been recorded as arising directly from the toxicity of these drugs, some investigators feel there is evidence that fatalities from severe side-effects occur. The manufacturers of the checkpoint inhibitors warn clinicians and patients that there is a risk of a positive feedback loop forming in the immune system, causing the immune system to attack healthy organs leading to severe problems. Doctors need to be careful when giving these drugs to patients with autoimmune disease. There is also some concern that when patients take antibiotics in advance of cancer treatment, the effectiveness of checkpoint inhibitors could be reduced. Experts are calling for more research on this issue.
See also: http://jamanetwork.com/journals/jamaoncology/fullarticle/2174768
Dana-Farber Cancer Center made this video about checkpoint inhibitors:
The 2018 Nobel Prize for physiology or medicine was awarded to James Allison and Tasuku Honjo for their work with checkpoint inhibitors.
References:
https://www.nature.com/articles/s12276-018-0191-1
https://blogs.shu.edu/cancer/category/immunology-immunotherapy/checkpoint-inhibitors/
https://www.nature.com/articles/bjc2017434
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6454802/
 Today’s arsenal of chemotherapy agents includes many different classes of medicines. Researchers continue to find and test new drugs.
Today’s arsenal of chemotherapy agents includes many different classes of medicines. Researchers continue to find and test new drugs.