Interferons and interleukins for cancer treatment
In immunotherapy treatment, oncologists use antibodies, vaccines, immunotoxins, bacteria, immune cells, and cytokines to stimulate the immune system and to reduce the risk of cancer recurring. Currently, just two cytokines (IFNα and IL-2) are employed by doctors to treat cancer; other cytokines are under evaluation in various phases of preclinical and clinical trials.
Interferons and interleukins are members of the cytokine class – the regulatory or signaling biomolecules produced by the body’s immune system to act on the cells locally. Interferons (IFNα, IFNβ, and IFNγ) are proteins that help to fight infections and diseases. IFNα and IFNβ are generated by many of the body’s cells after viral infection and are involved in the innate immune response. IFNα and IFNβ increase the resistance of normal cells to natural killer (NK) cells and make cancer cells more vulnerable to killing by cytotoxic T cells. Interleukin-2 (IL-2) stimulates growth and activity of T cells. Lymphocytes treated with this interleukin dramatically enhance their antitumor activity.
Interferon alfa-2b (Intron® A), Interferon alfa-2a (Roferon® A)
These two drugs are synthetic versions of IFNα which is used to treat bladder cancer, melanoma, chronic myelogenous leukemia, multiple myeloma, hairy cell leukemia, Kaposi’s sarcoma, follicular non-Hodgkin’s lymphoma, condyloma acuminata, chronic myelogenous leukemia, kidney cancer, carcinoid syndrome, islet cell tumor, multiple myeloma, non-follicular non-Hodgkin’s lymphoma, cutaneous T-cell lymphoma, and desmoid tumor.
Scientists synthesize them by recombinant DNA techniques in bacteria E.coli with an inserted IFNα gene from human white blood cells.
How it works. IFNα increases the resistance of normal cells to NK cells, and makes cancer cells more vulnerable to killing by cytotoxic T cells. Interferon blocks growth and multiplication of cancer cells, and encourages cancer cells to send out substances that attract immune cells.
How it is given. Via injection under the skin, through a vein, into a muscle (3-7 times a week), and through the urethra and directly into the bladder (intravescially) for transitional cell carcinoma.
Using with other drugs. Sometimes it is used in a combination with bacilli Calmette-Guerin (BCG) to cure bladder cancer, with aldesleukin (Proleukin®,IL-2) to treat melanomas and kidney cancer.
Side effects. Flu-like symptoms, pain, redness, itching or swelling, hair thinning, fatigue, fever, dizziness, loss of appetite, weight loss, diarrhea, difficulty sleeping, lowering numbers of white blood cells, red blood cells, and platelets.
First approved by the FDA: 1986 but other formulations approved in other years. Approved for treatment of AIDS-related Kaposi sarcoma, Hairy cell leukemia, Melanoma, and Follicular lymphoma .
Aldesleukin (Proleukin®, IL-2)
This is a medicinal form of IL-2 used to treat kidney cancer and skin cancer (melanoma). It is produced by recombinant DNA techniques in bacteria E. coli with inserted an IL-2 gene from human white blood cells.
IL-2 inhibits the growth and proliferation of cancer cells and makes them more visible to the immune system. The drug encourages the growth of killer T cells and other immune cells. You often see this drug referred to as a biological response modifier as it changes the immune system.
How it is given. As an injection just under the skin (daily for 5 days then 2 days rest for 4 weeks), into a vein (every eight hours for up to 15 doses).
Use with other medicines. Sometimes it is used with Interferon alfa-2b (Intron® A) to treat melanoma and kidney cancer.
Side effects. Vulnerability to infection, anemia, fatigue, breathlessness or a cough, drop in platelets, bruising (nosebleeds, bleeding gums, tiny red spots or bruises on arms or legs), flu-like symptoms (fever, chills, headaches), aching muscles and joints, confusion, depression, extreme sleepiness, low blood pressure, low urine output, weight gain, swelling in ankles, legs and face, a skin rash, diarrhea, loss of appetite, difficulty sleeping, feeling anxious or confused, a sore mouth, redness, swelling and soreness at the injection site.
First approved by the FDA: 1992. Approved for treatment of melanoma and renal cell carcinoma
Nogapendekin alfa inbakicept-pmln (Anktiva)
First approved by the FDA: 2024. Approved for treatment of bladder cancer.
See also: checkpoint inhibitors
References
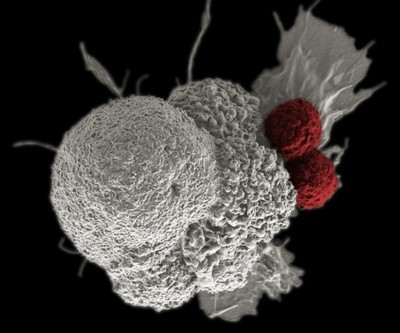
Cancer cell (white) being attacked by two cytotoxic T cells (red)
Aldesleukin. Lexicomp Online® [updated 2015 Sep 1; cited 2015 Sep 10]. Lexi-Drugs®. Hudson, Ohio: Lexi-Comp, Inc.; Sep10, 2015.
Coico R, Sunshine G, Benjamini E (2003) Immunology: a short course. 5th ed. Wiley-Liss. pp. 153, 154, 161, 279, 281-283
http://chemocare.com/chemotherapy/drug-info/il-2.aspx
http://chemocare.com/chemotherapy/drug-info/interferon-alfa.aspx
https://www.cancer.gov/publications/dictionaries/cancer-terms/def/interferon
https://my.clevelandclinic.org/health/treatments/interferons
http://www.cancer.org/cancer/melanoma-skin-cancer/treating/immunotherapy.html
http://www.mayoclinic.org/diseases-conditions/bladder-cancer/basics/treatment/con-20027606
http://www.mayoclinic.org/diseases-conditions/melanoma/basics/treatment/con-20026009
Lodish H (2008) Molecular cell biology. 1st ed. New York: W.H. Freeman. pp. 580-581
Maus MV. Immunology: T-cell tweaks to target tumours. Nature. 2017 Feb 22. doi: 10.1038/nature21506.
Proleukin (aldesleukin) https://proleukin.com/media/cjaawpxi/prl-hcp-brochure.pdf
Interferon
Brand/Trade Names: Intron A, Roferon A
Formula:
Manufacturers: Biotechnica Pharma Global, KinBio
Mechanism: Immunostimulant
Administration: Intravenous
Notes: Approved by FDA in 1986. Used for treatment of Karposi’s sarcoma, hairy cell leukemia, melanoma, and follicular lymphoma.
Aldesleukin
Brand/Trade Names: Proleukin
Formula:
Manufacturers: Prometheus Laboratories Inc., Guna spa
Mechanism: Immunostimulant
Administration: Intravenous
Notes: Approved by FDA in 1992. Used for treatment of melanoma and renal cell carcinoma.
 Today’s arsenal of chemotherapy agents includes many different classes of medicines. Researchers continue to find and test new drugs.
Today’s arsenal of chemotherapy agents includes many different classes of medicines. Researchers continue to find and test new drugs.