Anthracyclines
 Anthracyclines are anticancer drugs that were originally derived from Streptomyces bacteria. Their anti-tumor activity was established in the 1960s[1] and they were introduced into clinical use in the 1970s. Anthracyclines are red aromatic polyketides and occur in a variety of forms due to the structural differences in the aglycone base molecule and the different sugar residues attached[2]. These drugs are non-cell-cycle specific.
Anthracyclines are anticancer drugs that were originally derived from Streptomyces bacteria. Their anti-tumor activity was established in the 1960s[1] and they were introduced into clinical use in the 1970s. Anthracyclines are red aromatic polyketides and occur in a variety of forms due to the structural differences in the aglycone base molecule and the different sugar residues attached[2]. These drugs are non-cell-cycle specific.
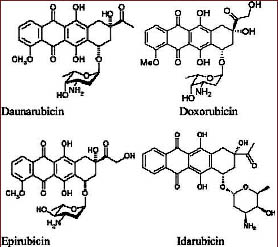
Daunorubicin and doxorubicin were early chemotherapy agents in this class. When doctors found that tumors developed resistance to those drugs that side effects, including cardiotoxicity, limited doses that patients could handle, medicinal chemists tried to find modifications of these drugs – analogs with wider activity and lower toxicity. More than 2000 analogs have been studied over the years in an effort to find better anthracyclines[6]. However, only very few anthracycline analogs like epirubicin and idarubicin[7] have been approved for clinical use. Cardiac toxicity remains a major concern when using anthracyclines.
You hear about how free radicals are bad in your body and how antioxidants are needed to stop radicals. But free radicals can also be useful in impairing DNA functioning in malignant cells. Anthracyclines actually generate free radicals that break DNA strands and thereby stop replication. They are not phase-specific and will work regardless of where the cell is in the reproductive cycle.
Major Drugs in the class
Daunorubicin
Daunomycin aka daunorubicin was the first anthracycline compound to be characterized structurally and stereochemically. Daunorubicin is used in treating acute lymphoblastic and myeloblastic leukemias.
Doxorubicin
Adriamycin (generic name doxorubicin) is similar to daunorubicin [3, 4]. First derived from the Streptomyces peucetiussoil fungus, it is now produced semi-synthetically. Doxorubicin is one of the most widely used chemotherapeutic agents and is generally prescribed in combination with other drugs. Doxorubicin has a broad spectrum of activity. It is sometimes called “red devil chemo” partly due to its red color.
It is one of the most effective drugs for solid tumor treatment, e.g., breast cancer, small cell lung cancer and ovarian carcinoma treatments. It has significant activity against bladder, stomach, liver and thyroid tumors, Ewings and osteogenic bone tumors, soft tissue sarcoma, neuroblastoma and Wilms tumor. It is also active against multiple myeloma, several types of leukemia and cutaneous T-cell lymphoma. It is also plays an important role in treatment of Hodgkins disease and non-Hodgkins lymphomas[5].
This medicine is notorious for severe side effects and everyone remarks on the bright red color, which even makes the patient’s urine red.
Epirubicin
Epirubicin is an epimer of doxorubicin and differs only in the orientation of the C-4 hydroxyl group on the sugar. Because of this slight change in the structure, epirubicin has lower cardiotoxicity than doxorubicin. Epirubicin is used in the treatment of gastric and breast cancer and is also indicated for the treatment of carcinoid, endometrial, lung, ovarian, esophageal and prostate cancers as well as soft tissue sarcomas [5].
Idarubicin
Idarubicin is an analog of daunorubicin. It lacks the C-4 methoxy group, and this increases its lipophilicity. Idarubicin has improved activity as induction therapy for acute myelogenous leukemia[5].
Valrubicin
Valrubicin is N-trifluoroacetyl, 1-4-valerate derivative of doxorubicin. Valrubicin enters cells more rapidly than doxorubicin. It is used specifically in the treatment of early bladder cancer [8].
Mechanism of action
Anthracyclines inhibit cancer through multiple pathways. They promote creation of free radicals which destroy nucleic acids and other biomolecules. They inhibit synthesis of DNA. Some anthracyclines appear to inhibit the topoisomerase II enzyme.
Anthracyclines are considered non-cell specific drugs. They are used on a wide range of cancers. Their major drawback is toxicity on heart muscle.
Accumulation of anthracyclines in the nucleus of neoplastic and proliferating cells
Anthracyclines enter the cells through passive diffusion[9]. An elegant mechanism of the selective transport of anthracyclines to the nuclei of neoplastic and proliferating cells has been proposed[10, 11]. It has been demonstrated that after doxorubicin molecules enter cells, they bind with proteasomes in the cytoplasm. The drug-proteasome complex is then translocated into the nucleus. Proteasomes are located predominantly in the nucleus of neoplastic and normal proliferative cells in contrast to non-proliferative normal cells in which proteasomes are predominantly in the cytoplasm[12-14]. That’s why there is a relatively higher rate of transport of anthracyclines into the nucleus of the neoplastic cells than into most non-malignant cells, and that’s why these drugs can be effective anti-cancer agents. Once the anthracyclines reach the nucleus they dissociate from the proteasome and bind to DNA. Moreover, binding of anthracyclines to proteasomes also inhibits the protease activity leading to inhibition of degradation of proteins involved in cell growth and metabolism and thus inducing apoptosis of these cells.
DNA intercalation
Intercalation into DNA leading to inhibition of macromolecular synthesis was the first mechanism described for cytotoxicity of anthracyclines[15]. The strong binding of daunorubicin and doxorubicin to DNA has been characterized extensively [16-18]. However, it has been observed that other anthracyclines, such as those of the nogalamycin family, that the antitumor activity correlates with a decrease in affinity for DNA [19, 20]. Considering this and also taking into account that the DNA in cells does not occur naked but as chromatin, it seems unlikely that DNA intercalation is the only or most essential pathway of anthracycline cytotoxicity.
On the other hand, anthracyclines like doxorubicin at low concentrations have been shown to selectively displace nuclear proteins[21], and daunorubicin has been shown to induce aggregation of chromatin[22]. This mechanism involves initial intercalation of the drug into the linker regions where the DNA is free of nuclear proteins, leading to conformational changes in DNA that extend towards the histone octamer and result in the unfolding of chromatin and its subsequent aggregation[22].
Interaction with DNA binding proteins
Regulation of gene expression by inhibiting, or promoting, the binding of transcription factors is also thought to play a role in anthracycline cytotoxicity with the potential involvement of SP-1 transcription factor as a specific target for these drugs [23, 24]. Involvement of anthracyclines in inhibiting DNA synthesis by affecting the initiation or the elongation phase, and RNA synthesis by inhibiting RNA polymerase activity has also been documented[25-27]. Another anthracycline mechanism that has intrigued scientists is activity as a Topoisomerase II poison[28]. Anthracycline rings that do not intercalate into the DNA seem to play a role in stabilizing the complex between Topoisomerase II and the DNA that it has nicked. The DNA nicks cannot be sealed and this leads to an accumulation of DNA damage that is cytotoxic due to growth arrest in G1 and G2 and programmed cell death[29]. Doxorubicin and Idarubicin have also been shown to inhibit Topoisomerase I and this is proposed to be an ancillary mechanism of cytotoxic activity of anthracyclines[30].
Anthracyclines, p53 and apoptosis
Like other genotoxic agents, doxorubicin has been demonstrated to induce the binding of p53 to DNA. The protein p53 is a major player in some forms of apoptosis, it has been proposed that anthracyclines may exert their cytotoxic effect via p53-mediated apoptosis. There are contradictory reports regarding this link between anthracyclines, p53 and apoptosis[29, 31, 32]. It is observed that there are more DNA breaks in p53 proficient cells than in p53 deficient cells although the levels of Topoisomerase II are same in the two cell types. It is therefore also proposed that p53 exerts this activity by binding to Topoisomerase II and inhibiting its ligase activity[33, 34]. However, clinical concentrations of these drugs induce apoptosis pathways that do not always require p53 by triggering a cyclic cascade of sphingomyelin hydrolysis and formation of ceramide[35]. It is also observed that anthracyclines can release cytochrome C from mitochondria directly and induce apoptosis[36]. Thus, although p53 seems to play some role in the activity of anthracyclines, it is not necessarily the only mechanism.
Free radical generation
One electron addition to the quinone moiety in ring C of anthracyclines leads to formation of semiquinone that regenerates back to quinone by reducing oxygen to reactive oxygen species like superoxide anion and hydrogen peroxide. The semiquinone can oxidize with the bond between ring A and daunosamine which results in deglycosylation. The aglycone thus formed has higher solubility in lipids and can intercalate into cell membranes and form reactive oxygen species which can affect sensitive targets[37, 38]. The one electron redox cycle of doxorubicin has been demonstrated to induce the release of iron from the stores. Doxorubicin forms a complex with iron, and this complex is capable of producing hydroxyl ions which are a more potent reactive oxygen species[39, 40]. Thus, oxidative damage plays a role in the mechanism of anthracycline activity. However, as the production of measurable reactive oxygen species is predominantly observed at supraclinical concentrations, this may not be the direct mechanism of anthracycline activity. Reactive oxygen species, though, can act as signaling molecules at very low, unmeasurable concentrations and induce apoptosis, and this could be one of the mechanisms by which anthracyclines exert their cytotoxic effect.
Antiangiogenic mechanism
Scientists have found that the anthracyclines inhibit transcriptional factor HIF-1 from binding to DNA in hypoxic human cells and inhibit tumor growth in human prostate cancer xenografts. Inhibition of HIF-1 transcriptional activity leads to decreased VEGF, SDF1 and SCF expression because of which there is decreased CAC mobilization and this results in decreased tumor vascularization and growth. Thus, anthracyclines can also inhibit cell growth through antiangiogenic pathways.
Side Effects
The side effects of anthracyclines, like other conventional chemotherapeutic agents, are linked to their cytotoxicity to non-malignant, proliferating normal cells. These side effects include nausea, vomiting, and alopecia. However, the major toxicities of anthracyclines include cardiotoxicity and myelosuppression, and these are the major limitations of these drugs. Doxorubicin can also cause severe local tissue necrosis. Cardiomyopathy and congestive heart failure are the two cardiotoxic side effects of anthracyclines. Epirubicin is less cardiotoxic than doxorubicin but may not totally eliminate the risk of chronic cardiotoxicity[5].
Anthracycline-induced cardiotoxicity is irreversible and is thus an especially important consideration in the treatment of curative malignancies in pediatric patients[4. The interaction between anthracyclines and iron[ has been found to play a role in anthracycline-induced cardiomyopathies by producing potent reactive oxygen species. An iron chelator – dexrazoxane – can be given as a chemoprotectant to patients who are prescribed high doses of doxorubicin to prevent cardiotoxicity.
Other strategies to limit the cardiotoxic effects of anthracyclines are also being employed that include limiting the overall dosage, encapsulation into liposomes, combination treatment, use of cardioprotector medications, and synthesis of less harmful modified anthracyclines[5].
PDF List of anthracyclines.
Allergic reactions
Although rare, mild to medium allergic reactions have been reported for anthracyclines. The symptoms include generalized urticarial exanthema as reported for epirubicin and doxorubicin. High doses of epirubicin can cause high fever, hypertension and hypoxia as hypersensitivity symptoms. Pegylated and liposomal doxorubicin and daunorubicin that have lower toxicity have been reported to produce acute hypersensitivity infusion reactions.
References
1. Brockmann, H., [Anthracyclinones and Anthracyclines. (Rhodomycinone, Pyrromycinone and Their Glycosides)]. Fortschr Chem Org Naturst, 1963. 21: p. 121-82.
2. Anthracycline Chemistry and Biology: Biological Occurence and Biosynthesis, Synthesis and Chemistry: No. 1. 1 (9 July 2008) ed. Topics in Current Chemistry, ed. K. Krohn: Springer.
3. Arcamone, F., et al., Adriamycin (14-hydroxydaunomycin), a novel antitumor antibiotic. Tetrahedron Lett, 1969. 13: p. 1007-10.
4. Arcamone, F., et al., Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol Bioeng, 1969. 11(6): p. 1101-10.
5. Cortes-Funes, H. and C. Coronado, Role of anthracyclines in the era of targeted therapy. Cardiovasc Toxicol, 2007. 7(2): p. 56-60.
6. Minotti, G., et al., Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev, 2004. 56(2): p. 185-229.
7. Arcamone, F., Properties of antitumor anthracyclines and new developments in their application: Cain memorial award lecture. Cancer Res, 1985. 45(12 Pt 1): p. 5995-9.
8. Kuznetsov, D.D., et al., Intravesical valrubicin in the treatment of carcinoma in situ of the bladder. Expert Opin Pharmacother, 2001. 2(6): p. 1009-13.
9. Skovsgaard, T. and N.I. Nissen, Membrane transport of anthracyclines. Pharmacol Ther, 1982. 18(3): p. 293-311.
10. Kiyomiya, K., S. Matsuo, and M. Kurebe, Mechanism of specific nuclear transport of adriamycin: the mode of nuclear translocation of adriamycin-proteasome complex. Cancer Res, 2001. 61(6): p. 2467-71.
11. Kiyomiya, K., et al., Correlation between nuclear action of anthracycline anticancer agents and their binding affinity to the proteasome. Int J Oncol, 2002. 21(5): p. 1081-5.
12. Kumatori, A., et al., Abnormally high expression of proteasomes in human leukemic cells. Proc Natl Acad Sci U S A, 1990. 87(18): p. 7071-5.
13. Amsterdam, A., F. Pitzer, and W. Baumeister, Changes in intracellular localization of proteasomes in immortalized ovarian granulosa cells during mitosis associated with a role in cell cycle control. Proc Natl Acad Sci U S A, 1993. 90(1): p. 99-103.
14. Palmer, A., et al., Changes in proteasome localization during the cell cycle. Eur J Cell Biol, 1994. 64(1): p. 163-75.
15. Marco, A. and F. Arcamone, DNA complexing antibiotics: daunomycin, adriamycin and their derivatives. Arzneimittelforschung, 1975. 25(3): p. 368-74.
16. Chaires, J.B., N. Dattagupta, and D.M. Crothers, Studies on interaction of anthracycline antibiotics and deoxyribonucleic acid: equilibrium binding studies on interaction of daunomycin with deoxyribonucleic acid. Biochemistry, 1982. 21(17): p. 3933-40.
17. Barcelo, F., et al., Equilibrium binding of daunomycin and adriamycin to calf thymus DNA. Temperature and ionic strength dependence of thermodynamic parameters. Biochem Pharmacol, 1988. 37(11): p. 2133-8.
18. Chaires, J.B., et al., Parsing the free energy of anthracycline antibiotic binding to DNA. Biochemistry, 1996. 35(7): p. 2047-53.
19. Krueger, W.C., et al., The interaction of nogalamycin and analogs with DNA and other biopolymers. Chem Biol Interact, 1981. 36(1): p. 1-18.
20. Bhuyan, B.K., et al., Comparative genotoxicity of adriamycin and menogarol, two anthracycline antitumor agents. Cancer Res, 1983. 43(11): p. 5293-7.
21. Bartkowiak, J., et al., Selective displacement of nuclear proteins by antitumor drugs having affinity for nucleic acids. Proc Natl Acad Sci U S A, 1989. 86(13): p. 5151-4.
22. Rabbani, A. and J. Davoodi, Effects of anthracycline antibiotic, daunomycin on thymus chromatin: the role of chromosomal proteins. Gen Pharmacol, 1994. 25(4): p. 787-93.
23. Gniazdowski, M., et al., Effects of anticancer drugs on transcription factor-DNA interactions. Expert Opin Ther Targets, 2005. 9(3): p. 471-89.
24. Mansilla, S. and J. Portugal, Sp1 transcription factor as a target for anthracyclines: effects on gene transcription. Biochimie, 2008. 90(7): p. 976-87.
25. Fritzsche, H., et al., Anthracycline antibiotics. Interaction with DNA and nucleosomes and inhibition of DNA synthesis. Biochemistry, 1987. 26(7): p. 1996-2000.
26. Studzian, K., et al., Inhibition of RNA synthesis in vitro and cell growth by anthracycline antibiotics. Neoplasma, 2001. 48(5): p. 412-8.
27. Leng, F. and G.H. Leno, Daunomycin disrupts nuclear assembly and the coordinate initiation of DNA replication in Xenopus egg extracts. J Cell Biochem, 1997. 64(3): p. 476-91.
28. Tewey, K.M., et al., Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science, 1984. 226(4673): p. 466-8.
29. Perego, P., et al., Role of apoptosis and apoptosis-related genes in cellular response and antitumor efficacy of anthracyclines. Curr Med Chem, 2001. 8(1): p. 31-7.
30. Guano, F., et al., Topoisomerase poisoning activity of novel disaccharide anthracyclines. Mol Pharmacol, 1999. 56(1): p. 77-84.
31. Ruiz-Ruiz, C., et al., Characterization of p53-mediated up-regulation of CD95 gene expression upon genotoxic treatment in human breast tumor cells. J Biol Chem, 2003. 278(34): p. 31667-75.
32. Inoue, A., et al., Administration of wild-type p53 adenoviral vector synergistically enhances the cytotoxicity of anti-cancer drugs in human lung cancer cells irrespective of the status of p53 gene. Cancer Lett, 2000. 157(1): p. 105-12.
33. Cowell, I.G., et al., Human topoisomerase IIalpha and IIbeta interact with the C-terminal region of p53. Exp Cell Res, 2000. 255(1): p. 86-94.
34. Dunkern, T.R., et al., Resistance of p53 knockout cells to doxorubicin is related to reduced formation of DNA strand breaks rather than impaired apoptotic signaling. DNA Repair (Amst), 2003. 2(1): p. 49-60.
35. Laurent, G. and J.P. Jaffrezou, Signaling pathways activated by daunorubicin. Blood, 2001. 98(4): p. 913-24.
36. Green, P.S. and C. Leeuwenburgh, Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim Biophys Acta, 2002. 1588(1): p. 94-101.
37. Gille, L. and H. Nohl, Analyses of the molecular mechanism of adriamycin-induced cardiotoxicity. Free Radic Biol Med, 1997. 23(5): p. 775-82.
38. Licata, S., et al., Doxorubicin metabolism and toxicity in human myocardium: role of cytoplasmic deglycosidation and carbonyl reduction. Chem Res Toxicol, 2000. 13(5): p. 414-20.
39. Minotti, G., G. Cairo, and E. Monti, Role of iron in anthracycline cardiotoxicity: new tunes for an old song? Faseb J, 1999. 13(2): p. 199-212.
40. Myers, C., The role of iron in doxorubicin-induced cardiomyopathy. Semin Oncol, 1998. 25(4 Suppl 10): p. 10-4.
41. Lee, K., et al., Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc Natl Acad Sci U S A, 2009. 106(7): p. 2353-8.
42. Von Hoff, D.D., et al., Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med, 1979. 91(5): p. 710-7.
43. Cvetkovic, R.S. and L.J. Scott, Dexrazoxane : a review of its use for cardioprotection during anthracycline chemotherapy. Drugs, 2005. 65(7): p. 1005-24.
44. Shepherd, G.M., Hypersensitivity reactions to chemotherapeutic drugs. Clin Rev Allergy Immunol, 2003. 24(3): p. 253-62.
45. Oltmans, R. and S.G. van der Vegt, Serious allergic reaction to administration of epirubicin. Neth J Med, 2003. 61(6): p. 226-7.
46. Solimando, D.A., Jr. and J.P. Wilson, Doxorubicin-induced hypersensitivity reactions. Drug Intell Clin Pharm, 1984. 18(10): p. 808-11.
 Today’s arsenal of chemotherapy agents includes many different classes of medicines. Researchers continue to find and test new drugs.
Today’s arsenal of chemotherapy agents includes many different classes of medicines. Researchers continue to find and test new drugs.