Drug resistance in chemotherapy treatment
One obstacle to successful chemotherapy treatment is drug resistance. Patients receiving chemotherapy can develop resistance to previously effective drugs to the point that the medicines no longer function as intended. Resistance – also called tachyphylaxis – occurs when a cancer cell develops the ability to keep the chemotherapy drug from entering it, or reduces the amount that can enter to a level that does not cause damage. Tolerance and desensitization are terms also used to describe growing resistance to drugs. (Tachyphylaxis is more precisely quickly acquired resistance while desensitization implies a slower process.) The cancer is said to be refractory when there is no response to a drug that normally works for that cancer.’
Sometimes a distinction is made between primary resistance, which is when the cancer cells have resistance to the medicine before they encounter it, and acquired resistance, which is when the tumor develops a resistance in the face of the chemotherapy drug. For acquired resistance to develop, the tumor essentially evolves. The point in treatment at which resistance occurs depends largely on the type of cancer being treated.
Resistance plays a big part in oncologists’ decisions about dose sizes. The amplitude of response to chemotherapy is limited by tumor cell resistance.
Mechanisms
Cancer phenotypes can adapt to therapeutic perturbations. This is effectively evolution on a very small scale, leading to and a consequence of temporal and spatial heterogeneity of the tumor microenvironment.
Many mechanisms cause or contribute to the development of resistance at a biochemical level. These include cell death inhibition, efflux, metabolic destruction of medicine, mutation of target genes, and the transition of the cells to mesenchymal stem cells, which is a process involved in the start of cancer metastasis.
In the 1970s scientists developed the Goldie–Coldman hypothesis, which postulated that every solid tumor is to some extent heterogeneous (has different phenotype cells), that there was always a chance that some of the mutant cells could survive a chemotherapy medicine, and that the bigger the tumor was the greater the fraction of cells were resistant.
Drug efflux is carried out by the ATP-binding cassette (ABC) transporter proteins. These proteins can pass through cell membranes and function to move compounds both into and out of the cell. The ABC proteins prevent the build-up of toxins in cells, and oncology drugs could be considered toxins. Multidrug resistance protein 1 (MDR1) – aka p-glycoprotein – and breast cancer resistance protein (BCRP) are ABC proteins known to play a part in removing chemotherapy drugs from cells. Scientists have found that the gene for MDR1 is expressed more when a person receives chemo treatment. The ABC proteins have been shown to remove kinase inhibitors and taxanes from the insides of cells.
Drug inactivation happens for all drugs, and the pharmacokinetic models take it into account. The body tends to get rid of foreign chemicals. Drugs are metabolized and that makes them ineffective.
DNA repair is when the cell fights back and fixes damage to the chromosomes done by chemotherapy agents. In normal healthy operation of the body, DNA damage response (DDR) mechanisms are present to maintain genomic integrity. Alkylating agents which act by damaging DNA, are particularly prone to resistance, and when a person is given them for a long time, the tumor cells can become 10 to 20 times more resistant.
Loss of hormone receptors occurs as we get older. It results in resistance to hormone therapy.
Small-scale evolution inside the tumor
Another dynamic – or perhaps a different way of looking at the mechanics of resistance – comes from the heterogeneity of the tumor. Not all cells in the tumor are the same or react the same in the face of a chemotherapy. Even if we assume the cancer started from a single cell and grew clonally, mutations that occur lead to the creation of malignant cells that resist a given chemotherapy drug. The Goldie–Coldman model of tumors assumes cells mutate and develop resistance at a rate proportional to “their intrinsic genetic instability.” This is another rationale for giving chemotherapy in combination regimens.
Chemotherapy drugs target characteristics that are different in malignant cells from healthy cells but even so, healthy cells are affected by the drugs. A big enough dose of the chemo agent would eliminate the cancer cells, but would kill so many healthy cells the patient might die. The therapeutic window is narrow. To cope with this problem, doctors administer chemotherapy (and radiation treatment) in cycles – repeated administration at smaller doses so the drug doesn’t kill the patient.
Unfortunately this approach does not kill all of the cancer cells. Sometimes it works as an effective therapy, but sometimes malignant cells survive and give rise to a newly hardened tumor.
Fibroblasts that are not malignant are induced by the chemotherapy to secrete Wnt16B. This is a protein that is taken up by the malignant cells and makes them hardier. What this means is that even non-malignant cells work to protect the tumor in the face of an assault by chemotherapy. If a patient receives drugs in a dose-dense cycle, it might lead to more resistant tumors in the future.
Drug designers need to worry about not just the cancerous cells but also the surrounding cells and microenvironment inside the tumor. Indeed, scientists are now looking into ways to block Wnt signaling.
Preliminary animal studies have shown that inhibiting WNT16B in prostate fibroblast cells slows tumor growth and makes cytotoxic drugs more effective.
Sanctuary Sites
If metastasizing cells get to certain parts of the body, they are more likely to survive cancer treatment, including chemotherapy. These areas are called sanctuary sites, and the brain and testes are common sites where cancer survives.
Born that way or made?
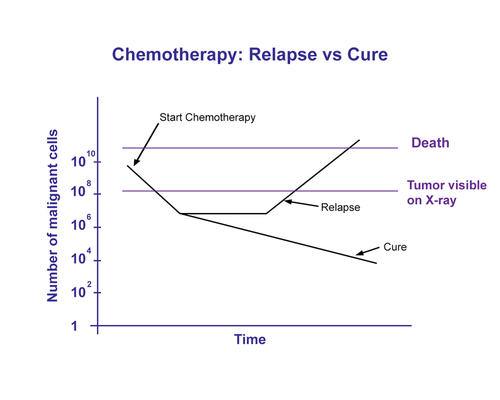
Are tumors naturally resistant to chemo medications, or do they acquire resistance during the course of the treatment? Both. The principles of evolution apply to cells in a tumor just as they apply to individuals. A chemotherapy treatment may kill some fraction of malignant cells. That fraction may be high, but it is not 100 percent. Some cancer cells remain even if the patient no longer has cancer in a clinical sense – the remaining tumor may be so small it cannot be detected. In some cases the patient’s immune system is able to control what’s left, and the patient can live for decades with the cancer in remission. vIn other cases, the remnant of malignant cells roars back as a cancer that is resistant to the chemotherapy employed.
Let’s say 0.5 percent of cells had a natural resistance and the initial chemo regimen destroyed 99 percent of the malignant cells. After that regimen, 50 percent of the remaining cells have resistance. When the tumor grows back, half of the cells now cannot be affected with chemo. Another round of medicine now kills only half of the tumor cells, and when the tumor grows back, it is now resistant to the chemotherapy agent. Medicine has therefore forced the evolution of the malignant cells within the tumor.
Oncologists are interested in how similar cells in a tumor are. All other things being equal, homogeneous tumors – groups of cells that are malignant but all alike each other – are easier to treat than heterogeneous tumors. Heterogeneous tumors contain cells that have many different mutations. Even though all the cancer cells can multiply and produce clinical cancer, the cells are cancerous in different ways. So a treatment could be effective on some or most cells in the tumor could be killed and the tumor size reduced even to a point of being non-deductible, but some malignant cells remain and a higher proportion of cells are heterogeneous.
How can we quantify how diverse a tumor is? Mathematicians have ways to do this. For a population (collection of individuals) a high diversity index number means members are diverse in whatever dimension is being measured.
Multidrug Resistance
Combination chemotherapy is intended to overcome or bypass resistance, but what if the tumor develops resistance to both agents being used? This happens and is one of the most frustrating parts of treating cancer. An article in Nature called it “the principal limiting factor to achieving cures in patients with cancer.” Aside from mutations in the cancer genome similar to those that cause some monodrug resistance.
Progress in overcoming resistance?
Working with one form of lung cancer, Israeli researcher Yosef Yarden found how malignancies develop resistance. This lung cancer responded to initial chemotherapy treatment and appeared to go into complete remission before reappearing in patients months later. The second coming of the cancer resisted chemotherapy. Yarden’s researchers found the cancer “rewired a main internal communications line” and produced new receptors that responded to growth signals but not to the chemotherapy agent. It’s almost like the cancer is clever and has a mind of its own.
Chemotherapy drugs that are most often associated with resistance include paclitaxel, docetaxel, vinorelbine, vincristine, vinblastine, doxorubicin, daunorubicin, epirubicin, etoposide, teniposide, topotecan, dactinomycin, and mitomycin C. These drugs are used to treat a wide variety of cancers – solid tumors to lymphoma. These drugs also come from several different families of chemotherapy drugs, so the phenomenon of drug resistance is not confined to one family of cancer drugs or one type of cancer.
In order to combat resistance, chemotherapy drugs are often given in combination in the hopes that the cancer will fail to resist at least one of the drugs in the combination. Once a cancer has developed resistance to one type of drug, it is more likely to develop resistance to other drugs, making treatment more difficult. This is why it is important to determine the best possible drug combination and to use it first when the probability of resistance is lowest. Another interesting method of emerging interest – though it is not yet used widely – is administering the chemotherapy drug on a long regimen of low doses.
Research is ongoing as scientists try to find a way to combat resistance. European researchers are studying the action of an enzyme called TAK-1 as it relates to extreme resistance found in pancreatic cancer. A substance was developed that inhibits the TAK-1 enzyme, and when this substance was given to mice along with the usual resistance-prone drugs, significant tumor reduction was observed. Other research is focusing on a substance called tariquidar, which attaches itself to the p-glycoprotein, hindering its ability to remove chemotherapy drugs from the cancer cell.
Resistance is one reason for remission in cancer cases.
Measurable residual disease monitoring
For leukemia patients, doctors sometimes formally look for signs of cancer after treatment. Called measurable residual disease (MRD) monitoring, this process is intended to identify cases for which continuing drug therapy is warranted.
Treatment of acute lymphoblastic leukemia is effective with most patients showing complete remission, but relapse often occurs. Quantitative reverse transcription PCR (RT-qPCR) can be used to look for trace quantities of cells. The M in MRD can stand for either minimal or measurable.
MRD is still new and formal studies have not shown it is effective, although it seems unlikely to do harm.
References
https://ncbi.nlm.nih.gov/pmc/articles/PMC4190567/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3646244/
https://pubmed.ncbi.nlm.nih.gov/17496207/
https://www.nature.com/articles/nrc706
https://pubmed.ncbi.nlm.nih.gov/24060863/
https://www.msdmanuals.com/professional/hematology-and-oncology/principles-of-cancer-therapy/systemic-cancer-therapy
 Today’s arsenal of chemotherapy agents includes many different classes of medicines. Researchers continue to find and test new drugs.
Today’s arsenal of chemotherapy agents includes many different classes of medicines. Researchers continue to find and test new drugs.